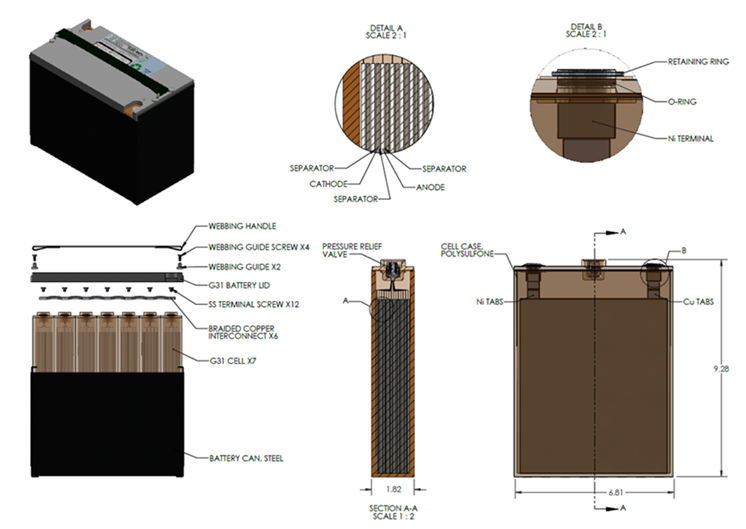
Our Technology
New market requirements and regulations are driving demand beyond what lead acid can provide. With over 30 years of development, ZAF has found a sustainable solution.

Why Nickel Zinc?
Ni-Zn batteries have excellent intrinsic properties, including high performance, long cycle life, low life-cycle cost, and low environmental impact. The breakthroughs achieved by ZAF include a proprietary electrolyte and zinc electrode formulation that greatly reduces zinc electrode solubility. These improvements permit long cycle life, high specific energy and specific power, along with maintenance-free operation. ZAF Ni-Zn batteries provide a viable and safe alternative to lithium-ion and lead-acid counterparts. Improved safety is one of the most significant advantages of the Ni-Zn battery, which makes this technology an ideal candidate for a variety of applications.
The Core Components of our Battery
Negative Electrode
ZAF’s negative electrode is primarily composed of zinc oxide that is doped with nucleation, migration stabilization, and hydrogen suppressant additives. The zincate nucleation additives are engineered to maintain a stable zinc structure throughout the life of the electrode. The migration stabilization additives work symbiotically with ZAF’s electrolyte to stabilize the zincate ion and the hydrogen suppressant additives minimize gassing, which reduces dry out in the battery.
Electrolyte
ZAF’s electrolyte is composed mostly of water, potassium hydroxide, and zinc stabilization additives. This novel electrolyte acts as the strands in a net encapsulating the zinc electrode. For the net to be effective, anchors must be engineered into the negative zinc electrode by way of the migration stabilization additives.
Positive Electrode
ZAF’s positive electrode is primarily composed of nickel hydroxide and conductive aids. Historically carbon has been used in large quantities in the positive electrode as a conductive aid; however, ZAF has been able to eliminate carbon from the electrode, thereby mitigating failure modes associated with carbon corrosion. The ZAF positive electrode is a very robust electrode with high utilization over a broad range of current densities.
ZAF Revolutionizes the NiZn Battery
With 30+ years of development, ZAF has found solutions that prepare the NiZn battery for the commercial market by mitigating failure modes associated with zinc shape change, dry out, and manufacturability.
ZINC SHAPE CHANGE
Historical Problem
In the Ni-Zn battery system zinc is partially soluble in the alkaline electrolyte and dissolves to form zincate anions. This process can lead to shape change, loss in capacity, and dendritic growth.
ZAF SOLUTION
ZAF’s engineered negative electrode contains nucleation and migration stabilization additives that work symbiotically with a novel electrolyte to stabilize the zincate ion. This mitigation strategy increases the cycle life of the Ni-Zn battery, while maintaining a greater amount of the initial capacity.
DFM
(Design for Manufacturing)
Historical Problem
In the past, Ni-Zn batteries were constrained to low volume applications due to the manufacturability of the chemistry. Subsequently, critical build parts (electrodes, for example) could not be scaled for high volume and automated manufacturing.
ZAF SOLUTION
The use of 3D current collectors and commonly utilized slurry casting techniques have enabled the Ni-Zn chemistry to be scaled from a low rate, handmade process to a high volume, reel-to-reel process.
DRY OUT
Historical Problem
A common failure mode encountered in the cycling of Ni-Zn cells, is the loss of electrolyte or dry out. This is caused by the production of oxygen and hydrogen from the decomposition of water.
ZAF SOLUTION
ZAF combats the dry out problem by integrating gassing suppressant additives into the negative electrode and incorporating a recombinant device into the battery. This recombinant device recombines the oxygen and hydrogen formed from the decomposition of water.
